Introduction
The human microbiome, an intricate constellation of microorganisms, plays a crucial role in health and disease, influencing a variety of disorders involving the skin and gastrointestinal (GI) tract.1 By modulating these microbes and their physiologic effects, potential therapeutic interventions can provide antimicrobial protection, enhance immunomodulation, and strengthen epithelial barrier maintenance.2 Emerging research on postbiotics—non-viable microbial cells and their components—suggests they offer health benefits similar to probiotics but without associated risk such as infections and gene transfer of antibiotic resistance. Unlike prebiotics, which are substrates utilized by host microorganisms, postbiotics encompass a range of substances produced by probiotics that can provide health benefits, offering a more tolerable and more stable therapeutic alternative.3 This paper focuses on the potential of postbiotics to modulate the gut-skin axis and treat skin disorders such as atopic dermatitis, leveraging their safe profile and robust efficacy.
Gut-Skin Axis
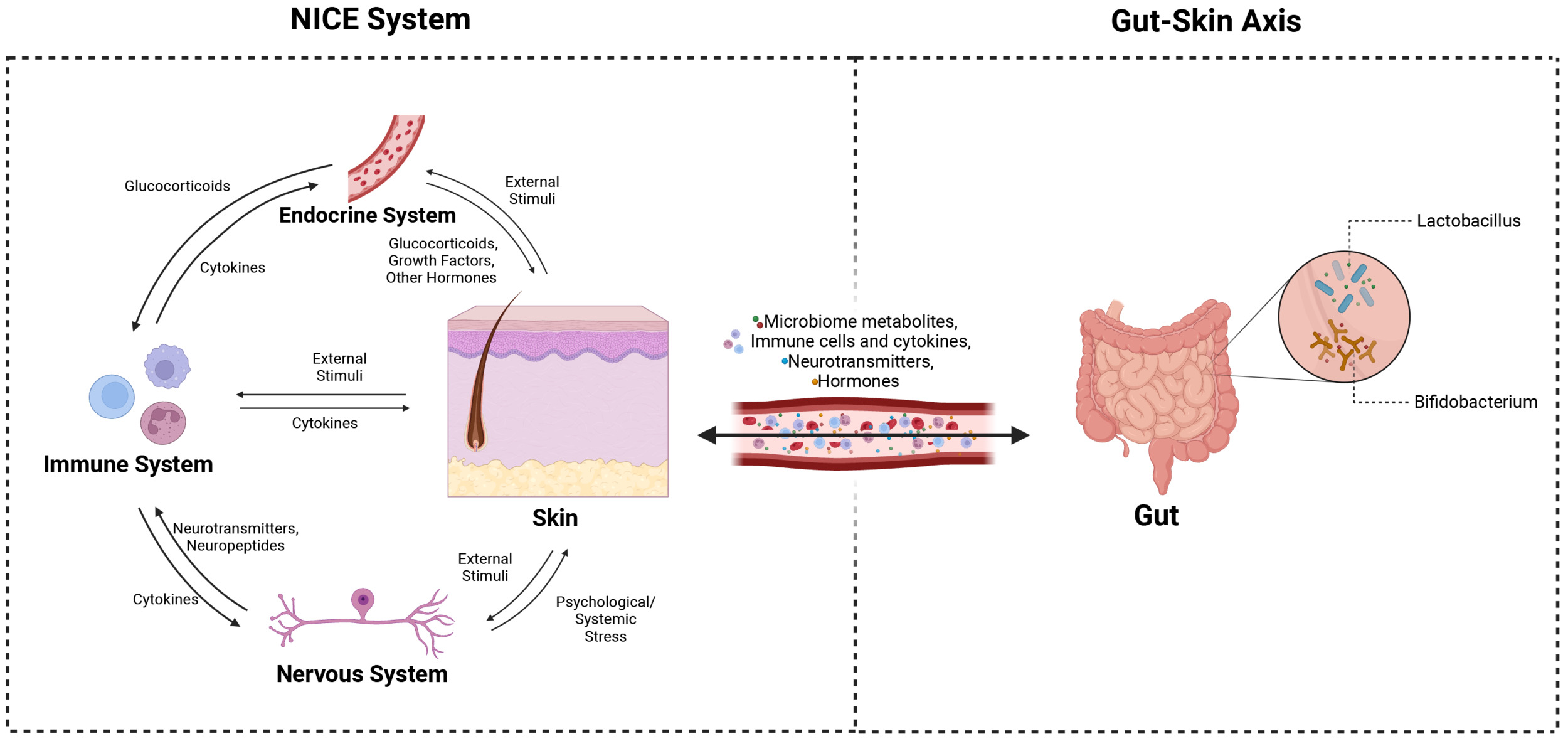
The gut-skin axis refers to the bidirectional communication between gut dysbiosis and skin homeostasis.4 Both the gut and the skin perform immunological and neuroendocrine functions, constantly adapting to environmental changes to maintain homeostasis.5 Each organ hosts a unique microbiome that enhances their respective immunoprotective functions, with systemic circulation enabling communication via immune cells, hormones, and metabolites. Therefore, dysbiosis in one can lead to disruptions in both, affecting their functions in immune protection.1,6
The key mediators of gut-skin communication interact to maintain this crosstalk. Specifically, this includes the role of immune cells like T cells and dendritic cells, as well as cytokines like IgA, in forming mucosal-associated lymphoid tissues (MALTs). These tissues protect against pathogens and inflammatory triggers, emphasizing their essential role in the immune system.5 Neuroendocrine mediators including cortisol, short-chain fatty acids (SCFAs), serotonin, dopamine, and GABA further modulate the gut-skin axis to regulate inflammation, itching, and barrier maintenance.1,7 Together, these dynamics constitute the neuro-immuno-cutaneous-endocrine (NICE) network, acting as the interface between epithelial dysbiosis and facilitating the gut-skin connection.8 (See Figure 1)
Probiotics and Postbiotics
Probiotics, live microorganisms that modulate the microbiome, increase populations of beneficial bacteria to regulate and strengthen the host immune response. Probiotics achieve such positive effects by introducing more quantities of beneficial bacteria already present.9 One of the most common strains of bacteria in the gut as well as probiotics is Bifidobacterium, known to promote gut health by its efficient carbohydrate and tryptophan metabolism. They are most notable for their use of dietary fiber for production of SCFAs, which are crucial for maintaining barrier integrity and modulating the inflammatory response.10 Another microbe commonly found in the gut and probiotics is Lactobacillus, which mainly generates lactic acid through its fermentation of refined sugars.11,12 Along with other microorganisms, these bacteria facilitate probiotic effects by clearing toxins, restricting the binding of pathogenic bacteria, and bolstering the immune response through their metabolites.9
When viable probiotic bacteria die, their cell components and metabolites remain bioactive and beneficial.13 This concept underpins postbiotics, the non-viable microorganisms and their nonliving byproducts that maintain health effects.2 These bioactive substances—including SCFAs, cell wall fragments, enzymes, and other metabolites—continue to exert anti-inflammatory, antioxidant, immunomodulatory, and antibacterial functions on the gut and thus improve overall health.14
One mechanism of postbiotics is protecting against pathogens. Postbiotics can compete with pathogens for adhesion sites, thus preventing their colonization. Certain metabolites, such as lactic acid and bacteriocins, also contribute by enhancing the antimicrobial properties against invasive bacteria and biofilms formed by pathogenic bacteria. Other mechanisms are strengthening the mucosal and epithelial barrier function and regulating immune responses. Through the modulation of certain cytokines (ie interleukins and TNF) and the elimination of free radicals, postbiotics exhibit anti-inflammatory and antioxidative effects, respectively.2
Atopic Dermatitis
AD is a chronic inflammatory skin disease characterized by pruritus, dry skin, eczematous patches, and lichenification. The pathophysiology centers around impaired skin barrier function.15 Consequently, irritants, allergens, and pathogens are able to cross the skin barrier, leading to inflammation and the classical symptoms of AD. The primary mechanism of inflammation in AD arises from an overactive Th2 response, leading to increased IL-4, IL-5, IL-13 and thus elevated IgE synthesis. The inflammation further disrupts the epithelial barrier and exacerbates the disease.15,16
Gut Microbiome & AD
Compared to healthy individuals, those with AD exhibit a gut dysbiosis characterized by reduced diversity and lower counts of Bifidobacterium and Lactobacillus.7 This altered microbial composition in the gastrointestinal tract causes decreased production of SCFAs, specifically acetate, propionate, and butyrate.10 These SCFAs maintain the barrier integrity of the gut and modulate inflammation and immune responses, suggesting a crucial function in influencing systemic health and AD exacerbations.17 SCFAs also directly influence the cutaneous microbial profile, serving as the pivotal link between changes in gut and skin microbial compositions.18 Individuals with AD often show increased levels of pathogenic bacteria, including Staphylococcus aureus, Clostridium difficile, and E. coli.19 These bacteria lead to immune dysregulation and increased intestinal permeability, worsening symptoms of AD by promoting toxin production and further gut barrier disruption.20
The interplay between gut dysbiosis and AD is mediated by the gut-skin axis and the NICE network. The relationship depends on an abnormal Th2 immune response and a dysfunctional hypothalamic-pituitary-adrenal (HPA) axis, which together attenuate the function of the NICE system in AD.21 This disturbance affects both the circulating immune compounds and the production of neuroendocrine metabolites within the gut that influence skin health. For example, tryptophan produced by the gut microbiome induces itch, a common symptom in AD. Interestingly, those with AD typically have lower levels of Bifidobacterium, which are key in the metabolism of tryptophan, resulting in higher levels of this amino acid and the associated itch.19
Other gut-derived neuroendocrine molecules in the skin contribute to the pathogenesis and severity of skin diseases, including AD. For instance, substance P, found in higher levels in individuals with AD, activates keratinocytes to produce growth factors and mast cells to release histamine, leukotriene, and tumor necrosis factor. These chemical mediators exacerbate skin inflammation, thus are crucial to the understanding of the pruritus seen in AD.22 Gut microbes significantly influence the levels of such neuropeptides, thereby regulating the downstream inflammatory mediators. Lactobacillus, for example, not only facilitates skin barrier recovery but also reduces skin inflammation related to substance P activity.23 This interaction emphasizes the complex communication between the gut microbiome and the skin via neuroendocrine factors, indicating that modulation of gut microbiota serves as a potential therapeutic target for managing AD.
These findings underscore that the gut microbiome activity influences both local and systemic host responses. This bidirectional communication facilitates the continuous feedback loop between the gut and the skin, impacting both organs’ barrier functions and microbiomes. Therefore, a disruption in the gut microbiome and subsequent skin barrier dysfunction contributes to the pathogenesis of AD.
Effects of probiotics and postbiotics on the treatment of AD
Probiotics target bacterial dysbiosis and the associated immune response to reduce flares of AD as well as systemic inflammation via the NICE network and the gut-skin axis.24 Postbiotics, nonviable cell metabolites, offer similar immunomodulatory effects with fewer risks of side effects compared to live bacteria.25–27
Heat-treated Lactobacillus strains significantly reduced the severity of AD in children aged 4-30 months.26 Progressive improvements in AD symptoms in adults treated with other strains of heat-killed Lactobacillus indicate their utility across various age groups.28,29
Interestingly, the efficacy of postbiotics in treating AD is influenced by both the bacterial strain and the preparation method. Tyndallization involves repeated cycles of heating and cooling, to prepare L. rhamnosus.30 Heat-killed Lactobacilli also offer protection from pathogens through bacteriocins that exhibit both antimicrobial and immunomodulatory effects.26,31 In individuals with AD, who are particularly susceptible to pathogenic bacteria, this defense mechanism is crucial for preventing overgrowth of microbes, like Staphylococcus aureus.24 However, it is important to recognize the limited number of studies on postbiotics. Emerging research demonstrates that heat-killed Lactobacillus strains modulate immune responses, regulating immune T cells and reducing IgE production in mice with AD.32
In addition to producing various neurotransmitters, such as GABA, serotonin, and dopamine, Lactobacilli secrete specific proteins, including protein p40, p75, and aggregation-promoting factor, aiding in the intestinal epithelial barrier integrity and promoting pathogen exclusion.25 This highlights the influence on the NICE system by targeting stress and the HPA axis. Since individuals with AD exhibit a dysfunctional HPA axis and altered NICE involvement, postbiotics show potential to alleviate AD symptoms. Bifidobacterium strains exhibit promising postbiotic potential in similar ways as Lactobacillus, by generating SCFAs, exerting antimicrobial effects, and regulating immune responses.2 (See Table 1)
By enhancing barrier function, down-regulating inflammatory markers, and protecting against pathogens, postbiotics offer a potential therapeutic approach to AD.
Conclusion
In contrast with live probiotics, postbiotics offer the promise of a safer approach for treating AD. These non-living microorganisms and their metabolites exert therapeutic actions through immunomodulation, strengthening of epithelial barriers, and protecting against pathogens. Specific strains, such as Lactobacillus and Bifidobacterium, and their components showcase the potential to influence the gut-skin axis as well as the NICE network.