Introduction
Chronic Actinic Dermatitis (CAD), also known as photosensitivity dermatitis, is broadly defined as dermatitis with or without a pseudolymphomatous eruption in sun-exposed regions.1 While this condition is rare, it occurs more often in older men, typically arising from a previously-diagnosed dermatitis background, including atopic, allergic contact, and seborrheic.2 While CAD most often arises in response to UV light, the condition can be misdiagnosed or missed altogether, especially in cases of widespread dermatitis.2
Case report
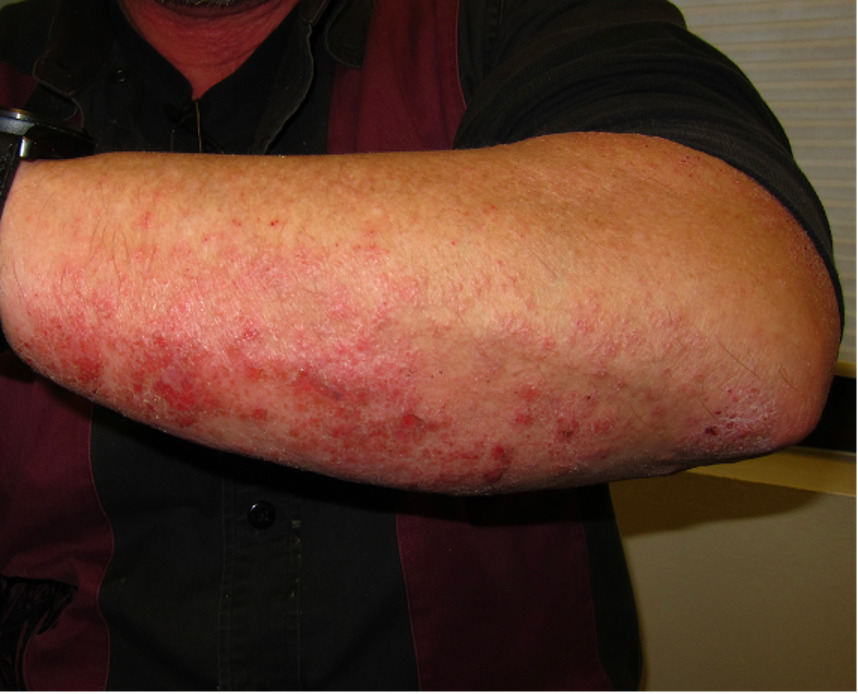
A 51-year-old Caucasian man with no significant medical history and not on any medications presented with a chief complaint of a long-standing itchy and painful rash almost exclusively n sun-exposed areas. The patient had eczematous patches with lichenification distributed on the anterior neck, upper chest, forearms, and both palmar and dorsal aspects of the hands. The rash was described as severe, with 17% body surface area involved. The patient worked in manual labor, spending long periods in the sun.
The patient reported that when he was extremely careful about both sunlight and visible light, using zinc oxide/titanium dioxide sunscreen, hats, and sun-protective clothing, he was able to heal to some degree, but upon even modest exposure to light, the rash would return, sometimes causing painful erosions and fissures that made it impossible for him to work. He was placed on disability due to his skin.
The previous diagnostic history of the patient included a negative skin patch testing and a negative antinuclear antibody assay. The patient had most recently been applying topical triamcinolone ointment regularly with minimal effect. The patient reported having previously tried multiple medications, including potent topical steroids in lotion and cream form, topical calcineurin inhibitors, topical and oral antifungals, oral mycophenolate, oral methotrexate, oral azathioprine, and oral apremilast with no success.
The broad differential diagnosis included severe atopic dermatitis, severe contact dermatitis, porphyria cutanea tarda, cutaneous T-cell lymphoma, cutaneous lupus erythematosus, and chronic actinic dermatitis.
A skin biopsy revealed histological features of epidermal spongiosis, lymphocyte exocytosis, and a superficial and deep perivascular lymphohistiocytic infiltrate, confirming the diagnosis of CAD.3 The remainder of the workup—including laboratory testing for lupus, porphyrias, cutaneous T-cell lymphoma, and patch testing—was unremarkable.
The patient was started on subcutaneous monotherapy dupilumab with a loading dose of 600mg and then 300 mg every two weeks. During the first two months of treatment with dupilumab, he experienced significant improvement of his eruption and was able to return to work. After approximately four months while on dupilumab, the patient experienced muscle aches thought to be related to dupilumab and discontinued therapy. However, at the start of summer, he experienced a flare of his CAD and decided to restart dupilumab, which quickly improved his symptoms and which he continues to tolerate. The patient continues to adhere to photoprotection and determined that the risk of muscle aches from dupilumab were outweighed by the excellent control of his CAD.
Discussion
While the pathophysiology of CAD is not fully understood, it is hypothesized that UV light damages DNA to create endogenous antigens, though how visible light may contribute is unclear.4 Subsequently, in a delayed hypersensitivity reaction similar to contact dermatitis, CD8+ cytotoxic T cells respond and damage the skin.3,5 Another theory suggests that patients with CAD have a preexisting abnormal sensitivity to UVA and UVB rays, resulting in characteristic erythema that arises in photo-sensitized regions. The increased sensitivity is also thought to be implicated in the development of dermatitis in response to fluorescent and other visible light sources which may emit little or no ultraviolet rays.3,4
Although there is no FDA-approved therapy for CAD, historical treatments include sun protection, topical steroids, and immunosuppressants.3 Additional recommended treatments include avoidance of known contact and photocontact allergens, as well as photosensitizing drugs, especially in patients with a history of contact dermatitis.3 Dupilumab is an IgG monoclonal antibody that inhibits interleukin-4 (IL-4) and interleukin-13 (IL-13).6 By inhibiting these proinflammatory cytokines, chemokines, and IgE antibodies are thus reduced.4 Notably, there have been case series and case reports outlining improvement of CAD after dupilumab, particularly in the Chinese population.7,8 We add this report to the literature.
Conclusion
CAD can be a debilitating disorder; this patient had to leave his job and take disability due to the outdoor nature of his job. Dupilumab represents a novel treatment that shows promise in treating CAD.7,8 More large-scale studies are needed to evaluate the full effect of dupilumab in treating CAD, both in terms of safety and efficacy, but clearly there is potential for this approach.
Funding Sources
No funding sources were secured for this study.
Disclosures
Dr. Lio reports research grants/funding from the National Eczema Association, AOBiome, Regeneron/Sanofi Genzyme, and AbbVie; is on the speaker’s bureau for Regeneron/Sanofi Genzyme, Pfizer, Eli Lilly, LEO, Galderma, and L’Oreal; reports consulting/advisory boards for Almirall, ASLAN Pharmaceuticals, Dermavant, Regeneron/Sanofi Genzyme, Pfizer, LEO Pharmaceuticals, AbbVie, Eli Lilly, Micreos, L’Oreal, Pierre-Fabre, Johnson & Johnson, Level Ex,Unilever, Menlo Therapeutics, Theraplex, IntraDerm, Exeltis, AOBiome, Realm Therapeutics, Altus Labs (stock options), Galderma, Amyris, Bodewell and My-Or Diagnostics.
KA reports no conflict of interest.